Myth #15: A synthetic molecule is different than the same molecule if made naturally.
That's right, you read it correctly, its not a mistake and I know its going to be hard for some of you to accept that this is in fact a myth, but I assure you it most certainly is. But, as always, there is more to this story that just leaving it there without further explanation and the truth really takes nothing away from the specialness of natural products and the complexity of essential oils over synthetic fragrances.
First, we need to accept and understand that individual molecules are not alive but merely three-dimensional arrangements of atoms to form unique structures in space, held together by shared electron densities that we call “bonds.” As such, an individual molecule has no "knowledge" of the pathway by which it was created and how it behaves in any system is a function of its three-dimensional structure, not of it’s origin. A molecule of say L-menthol (the main component of peppermint essential oil) will behave EXACTLY the same in any environment whether that molecule of L-menthol was made by the peppermint plant or the BASF Chemical plant. When 10 carbon atoms and 15 hydrogen atoms come together in the arrangement shown in the picture below we have L-menthol, regardless of who or what orchestrated the atoms coming together in this arrangement. L-menthol is L-menthol because of its structure. The structure is what defines the molecule, not its source.
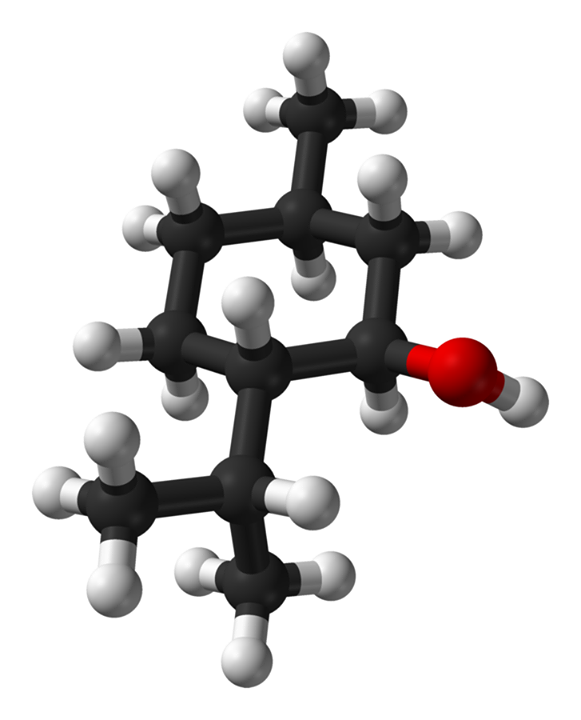
3D structure of L-Menthol - Carbons in black, Hydrogens in white and Oxygen in Red
Now, for the rest of the story. Yes its true that isolated individual molecules are universally the same regardless of who are what synthesized them. But this in no way means that essential oils can be re-constructed, molecule by molecule, in a lab. The reason this would be virtually impossible is because of the vast complexity of essential oils. Essential oils are almost always a collection of hundreds of molecules when you look at all the minor and trace components. The problem becomes infinitely more complex when you consider that almost every one of those components has an enantiomeric form to worry about as well. So, for example, while peppermint oil consists of 40-50% menthol and 99%+ of the menthol is the L form, there is also a small amount of its mirror image (D-menthol) in there as well. Not to mention that menthol has not one, but 3 chiral carbon atoms, so when you consider all of the diastereomers (things like iso-menthol, neo-menthol, neo-iso-menthol) along with their mirror images, there are a total of 8 menthol isomers to worry about! And this is just the molecular system of menthol (one out of close to hundred different compounds in peppermint) trying to exactly recreate the correct ratios of every enantiomer and/or diastereomer of every molecule in an essential oil would be a monumental task that is basically impossible from a practical standpoint.
So, the thing to take away from this is that there is nothing magical about a molecule simply because its made in "nature" (a meaningless term to me because what is not natural? Basically, if it exists its natural, you cannot separate man from nature). The "magic" if you will, is in the complexity that plants construct their COLLECTION of molecular mixtures that humans in a lab cannot even come close to exactly reproducing, at least not yet. Understanding the science doesn't it make it any simpler or less interesting, in fact, if you really understand whats going on, you realize that how incredibly complex and intricate things are and the more you know, the more you know you don't know about the universe. The complexity of nature is the real “magic,” not nature itself.