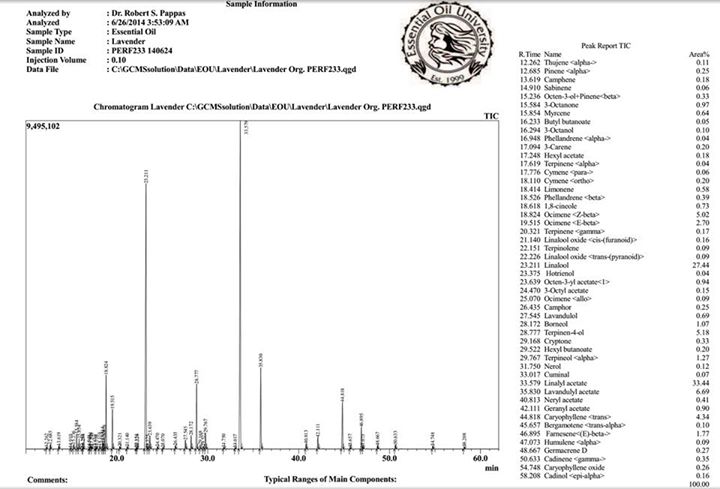
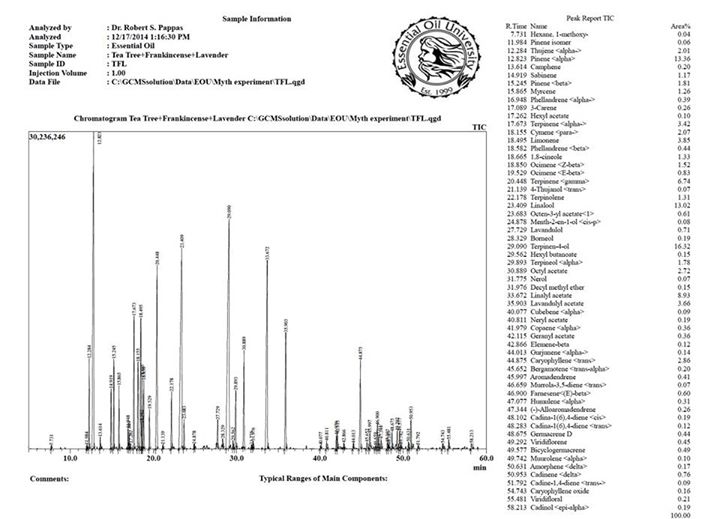
Estrogens
This is yet another claim apparently made by people who lack a fundamental understanding of chemistry and how molecules work in biological systems. As any sophomore biology or chemistry major in college knows, molecules in biological systems work the way they do because of VERY specific “hand-in-glove” type relationships between active molecules and receptor sites in the living systems. Molecules are three-dimensional structures and many times this perspective is lost to the untrained eye looking at two-dimensional representations of these structures on paper. The structure of the molecule basically determines whether or not it can perform the action of interest, this is why 3D computer molecular modeling is so vital in the drug discovery field. If a molecule doesn’t have the right shape and size to fit where it needs to fit, then its not going to be a candidate to meet the specific need that is being researched.
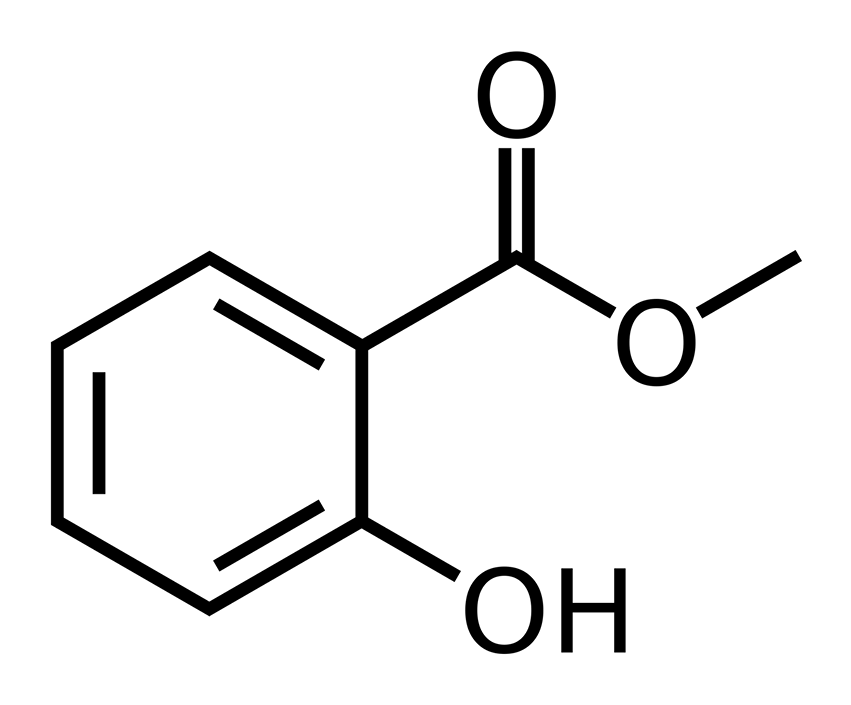
Having said the above, let’s look at some chemical structures and hopefully anyone can plainly see why its impossible for clary sage oil, or any essential oil for that matter, to act as an estrogen in the human body. There are three main estrogens in humans known as estrone or E1, estradiol or E2 and estriol or E3. All three of these molecules belong to the general class of molecules known as steroids. Steroids are defined by the four joined ring structures which include three six-membered rings and one five-membered ring arranged as in the structure shown below which is known as estradiol. Think of all steroids as three hexagons and one pentagon joined together, they must have this basic structure to be a steroid with the specific steroid of interest being defined by the various functional groups that are attached to this basic quad-ring system. Without this basic backbone structure, the molecule cannot be a steroid, nor can it behave as a steroid would in biological systems.
Estradiol, one of the three main estrogens, AKA E2.
So why is clary sage oil said to have estrogen like properties? It all has to do with a component found in the oil called Sclareol. So why is sclareol not a good candidate to have estrogen like properties? First of all sclareol is actually a very minute component of the essential oil of clary sage despite some authors claiming that sclareol is present in clary sage oil at 1.6-7.0%, an utterly ridiculous claim. Almost all steam distilled clary sage oils on the market (I would say 99.9% of them) have less than 0.5% sclareol content. As sclareol is a relatively heavy molecule, its really very difficult to get sclareol above that level with conventional steam distillation. To get the level higher, some proprietary distillation processes have to be implored and most companies will not go to that trouble because the sclareol is a valuable precursor to a very important molecule in the synthetic fragrance industry and not deemed important to the essential oil.
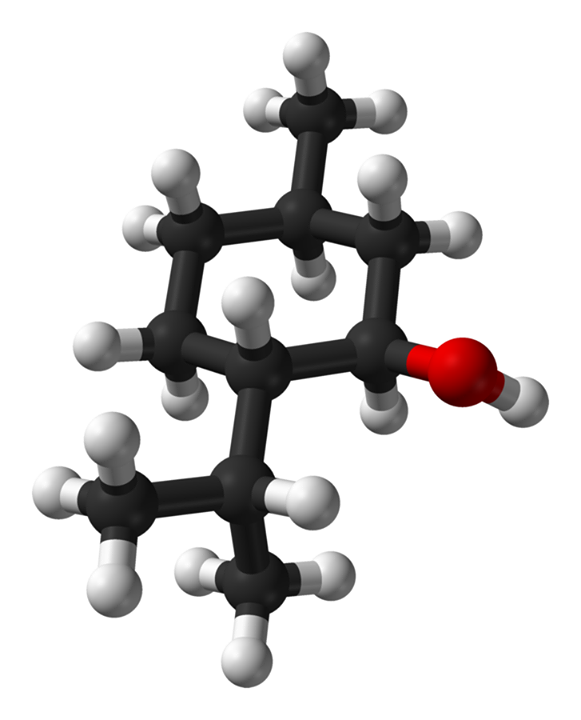
Secondly, if we look at the structure of sclareol shown below we will see that it actually has very little in common with the structure of any of the estrogen molecules, E2 being the one that it would have the most in common with because both are what we would call “diols” meaning there are two alcohol groups. Sclareol is not a steroid but what would have to be termed a diterpene diol, not even remotely close to the necessary steroidal backbone.
Sclareol, a diterpene idol found in clary sage oil in trace quantities.
In comparing the structure of sclareol with that of any of the steroidal estrogen structures we can easily see that sclareol lacks the quad-ring system that define steroids, in fact there are only 2 ring structures in sclareol, making it nowhere close to a good fit in any receptor site that would be accepting of any of the estrogens. Furthermore, the two alcohol groups in sclareol, which likely play a key role in how the estradiol molecule reacts once attached to its receptor, are in very different proximity to one another compared to estradiol. Remember, molecules in biological systems have very specific ways, based on three-dimensional structure, in which they interact to accomplish their designed tasks. Hopefully, even without a chemistry degree, anyone can see that, based on the structural parameters of both systems, there is no way that sclareol could ever perform the function of estrogen in the human body.
In summary, I think the chemical evidence is pretty clear that sclareol is not a steroidal estrogen, does not mimic the function of any estrogen molecules, does not stimulate estrogen production (why would it?), and would not appear to have any mechanism by which it can “balance hormones” at least not by a pathway that has anything to do with estrogens. If you see anyone making these types of claims, simply ask them to site the research that can propose a chemical mechanism that is remotely plausible to accomplish any of these tasks. I don’t think they will be able to produce anything credible to support the claims. If clary sage oil does actually work in any of the above capacities then it has to do it by some other mechanism, unrelated to how estrogens perform in the body. I am not saying that it’s impossible that clary sage can have some of the effects that have been claimed, but just be aware that its not really possible that the oil can mimic estrogens or that the oil contains estrogen like molecules.